 Summary of the problem
Summary of the problem
Heat stress is one of the biggest challenges the poultry industry is faced with in the tropical and even subtropical areas to get the advantages of the genetic advancements made in the recent past. While developing the modern breeds the selection pressure has been put on body weight and feed conversion efficiency and towards maximizing the yields of breast meat. While doing so the modern broiler and layer breeds have become extremely active from the metabolic point of view and this has decreased their thermal tolerance. The indirect impact of heat stress on the productivity of birds is quite discernible and the overall impact on the economics of farm operation is truly substantial. Hence, researchers engaged in poultry welfare, management and nutrition are constantly engaged in developing strategies to ameliorate the impacts of heat stress in chickens. Survivability and productivity of birds exposed to heat stress depend on the physiological integration of many organs and coordination between the neuroendocrine, gastrointestinal and immune systems is required which elicits the physiological, molecular, neuroendocrine and behavioural responses to cope up with the heat stress. Recent advancements and the application of molecular biology methodologies have allowed for a faster understanding of how birds do respond upon exposure to heat stress, and this has allowed researchers to develop more accurate techniques to mitigate the negative effects of heat stress with greater efficiency.
Ever increasing environmental temperature: a threat to the poultry industry
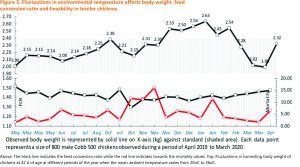
 Global warming is one of the major threats looming on human civilization. It is indeed very difficult to say what future the earth is awaiting even in 2050. For the domesticated and wild animals, the problem of excessive fluctuations in environmental temperature is a perennial problem which has just got confounded by the surge in environmental temperature and the genetic modifications made in the modern birds in the recent past. The poultry industry is facing threats due to a steady increase in environmental temperature. In the developed and even in many developing countries poultry birds are reared under controlled conditions under farmer’s supervision where the houses provide everything birds need to maintain their welfare and performance, including protection from the weather. However, several aspects of poultry production have changed through the 1990s and beyond 2000.The birds themselves, as stated above, have different genetic characteristics, they are much more productive, and there have been changes to medication and nutrition. The faster their growth rate the more they become susceptible to heat stress (Cahaneret al., 1995; Berong and Washburn, 1998). Peak summer temperatures outdoors even in many temperate countries have been recorded as high as 380C, and temperatures greater than 300Chave been occurring more regularly. Such upward changes in environmental temperature may cause failure in the environmental controlling systems of the poultry houses and impact bird’s performance especially when the ambient humidity goes too high. As a matter of fact, high ambient temperature and high relative humidity may be a critical, sometimes deadly combination for domesticated fowls and these effects are ought to intensify as global climate change is increasing both the annual average temperature and the frequency of high temperature extremes.
Global warming is one of the major threats looming on human civilization. It is indeed very difficult to say what future the earth is awaiting even in 2050. For the domesticated and wild animals, the problem of excessive fluctuations in environmental temperature is a perennial problem which has just got confounded by the surge in environmental temperature and the genetic modifications made in the modern birds in the recent past. The poultry industry is facing threats due to a steady increase in environmental temperature. In the developed and even in many developing countries poultry birds are reared under controlled conditions under farmer’s supervision where the houses provide everything birds need to maintain their welfare and performance, including protection from the weather. However, several aspects of poultry production have changed through the 1990s and beyond 2000.The birds themselves, as stated above, have different genetic characteristics, they are much more productive, and there have been changes to medication and nutrition. The faster their growth rate the more they become susceptible to heat stress (Cahaneret al., 1995; Berong and Washburn, 1998). Peak summer temperatures outdoors even in many temperate countries have been recorded as high as 380C, and temperatures greater than 300Chave been occurring more regularly. Such upward changes in environmental temperature may cause failure in the environmental controlling systems of the poultry houses and impact bird’s performance especially when the ambient humidity goes too high. As a matter of fact, high ambient temperature and high relative humidity may be a critical, sometimes deadly combination for domesticated fowls and these effects are ought to intensify as global climate change is increasing both the annual average temperature and the frequency of high temperature extremes.
The economic impacts of heat stress
Negative impacts of heat stress on poultry health and performance have been well characterized and include depressed growth, poor feed efficiency, decreased egg production, compromised immune function, disturbed intestinal integrity, and even lower survival rates(Lara and Rostagno, 2013). Heat stress is estimated to cost the poultry industry millions of dollars each year. This estimate would be even higher if the economic losses due to increased incidence of infectious diseases that the bird contract with due to oxidative damages occurring during exposure to heat stress could be included. The continuous increase in poultry production over the last decades to meet the high growing demand and provide food security has attracted much concern due to these recent negative impacts of the most challenging environmental stressor, heat stress on birds.
The physiological aspects of heat stress
The negative effects of heat stress range from reduced body weight gain, increased feed intake, and a poorer feed conversion ratio in birds (Awadet al., 2018) to poor meat quality (Sifaet al., 2018). Furthermore, heat-stressed broilers have been found to have negative impacts on caecal microbiota (Song et al., 2014). Acute heat stress causes the jejunum to lose weight and length (Mitchell and Carlisle, 1992) which suggests that heat stress directly affects the intestinal morphology to modify nutrient uptake.
The molecular basis of the effects of heat stress
Although reduced feed intake and body weight have been the key indicators of heat stress, deep insights into the modulation of nutrient uptake in heat-stressed chickens can be gained only by analysing the expression of the genes which are responsible for the transportation of nutrients from the luminal side to the systemic side. Similarly, to understand the basis of the damages caused by heat stress in the broiler or laying hens it is very much important to know and understand the molecular basis of the effects of heat stress which affects the expression of certain genes which are directly related to immunity and disease resistance (Al-Zghoulet al., 2019; Habashyet al., 2017). Recent breakthroughs in biotechnology have enabled researchers to investigate the consequences of heat stress at the molecular level. Evaluation of gene expression provides insights into how the cells respond to heat stress.
Effects on nutrient transporters genes
It has already been mentioned that high ambient temperature results in a decreased feed intake and compromised intestinal health in poultry across all the age groups and the effects are almost ubiquitous in all age groups. Exploring the response of the genes responsible for nutrient transportation across the mucosal membrane is an excellent technique to measure the impact of heat stress on gut health and understand its implications on nutrient uptake (Habashyet al., 2017; Sun et al., 2015).Glucose absorption during heat stress is crucial, as it is the main source of energy irrespective of the type of feed fed to the birds. It is primarily carried through sodium-dependent glucose transporter (SGLT) and glucose transporter (GLUT) systems in the epithelium and the non-epithelial cells, respectively. Under heat stress, glucose uptake gets reduced initially and then steadily remains at the decreased plane for the next several days. The sodium-independent glucose transporter (GLUT) systems include GLUT2 and GLUT5 genes – the former is responsible for transporting carbohydrates such as glucose, galactose, and fructose across the basolateral membrane and the latter controls the transportation of fructose across the brush border membrane (Uldryet al., 2004). Any factor that has negative effects (causing down-regulation) on the expression of these genes should have a negative effect on the absorption of glucose and other mono-sugars and thus should cause energy deficiency. This explains why broiler and laying hens suffer from growth depression and lower productivity when exposed to critically hot environmental temperatures. Heat stress down-regulatesthe expression of both the sodium-dependent and sodium independent glucose transporter genes along the length of the small intestine as could be observed with broiler chickens exposed to approximately 35oC for more than 10 days (Habashyet al., 2017;). With glucose absorption being severely impacted by heat stress, an extrapolation of the effect could be seen on the expression of insulin-like growth factor (IGF-1) gene. A negative correlation between the expression of the IGF-1 gene and ambient temperature has been reported (Gomes-Marcondes and Tisdale, 2002) which is accompanied by an increased expression of the genes related to protein degradation (Lee et al., 2012). The latter occurs as a compensatory mechanism to yield energy in order to keep the vital organs surviving during periods of heat stress.
Immune suppression during heat stress
Reduced humoral immunity is one of the most common forms of immunosuppression in heat-stressed chickens, which raises the risk of secondary infections and reduces immunisation efficiency (Lara and Rostagno, 2013; Jahanianet al., 2015). During heat stress, the number of peripheral blood lymphocytes decreases, and an electrolyte imbalance is induced (Borges et al., 2004). Spleen weight and macrophage activity also decrease (Bartlett and Smith, 2003), as does the number of CD4+ and CD8+ cells. Heat stress (31 and 36°C) applied for 10 hours a day between 35-42 d of age has been found to reduce peritoneal macrophage activity, increase corticosterone in serum, and induce modest alterations in intestinal mucosa in broiler chickens, indicating induction of an inflammatory process. These changes in heat-stressed birds show that broiler chickens have a neuroimmune connection. When chicks are exposed to high ambient temperatures, changes in the levels of immunological marker genes such as interleukins, toll-like receptors, and tumour necrosis factors have been observed in their spleen and intestine (Varasteh et al., 2005).
Heat and oxidative stress
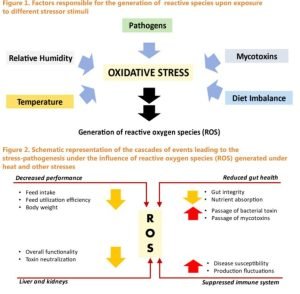
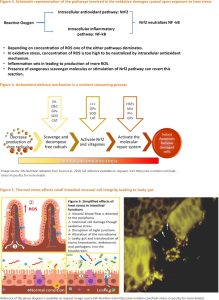
An increase in cellular energy demand appears to be the initial stage in the pathophysiology of HS. Yang et al. (2010) found that cells increase their energy expenditure by two folds after being exposed to extreme heat. After 6 hours of acute heat stress, Mujahid et al. (2006) found that mitochondrial transportation and oxidation of fatty acids were increased. The generation of reducing equivalents and the enzymatic activity of subunits of respiratory chain complexes are boosted to fulfil the increased energy demand of cells and mitochondrial biogenesis. Excessive formation of reactive oxygen species (ROS) promotes oxidative damage in avian muscle cells, resulting in lower body weight in heat-stressed hens (Mujahid et al., 2006).The over expression of the NADPH oxidase 4 gene and the down regulation of the avian uncoupling protein gene in muscle cells cause oxidative damage (Akbarianet al., 2016). The principal antioxidant systems that help the birds comba toxidative stress are superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPX). These antioxidant enzymes limit the excessive generation of ROS in a sequence of stages. Free radicals are broken down into hydrogen peroxide by SOD, while hydrogen peroxide is broken down into the water and molecular oxygen by CAT and GPX (Rimoldiet al., 2015). The use of gene expression profiles allows for a more precise strategic evaluation of modulations in response to temperature changes. Figures 1 and 2 depict the cascades of events which lead to the development of ROS and the generalised impact of ROS on the overall performance of chickens. In a simplistic way, it may be said that it is more important to have the redox reactions balanced at any given point in time. If the balance gets tilted more towards oxidative damages, then the antioxidant defence mechanisms step in and neutralize the negative effects of ROS. This pathway is mediated via the nuclear factor erythroid 2–related factor 2 (NrF2) pathway. However, in cases of excessive concentrations of ROS, the antioxidant defence mechanism fails and that leads to inflammatory reactions mediated via the nuclear factor kappa-light-chain-enhancer of activated B cells (NfKB) gene. The latter induces the production of more ROS and aggravates the already existing damages (Figure 3). Under such conditions, the presence of exogenous scavenger molecules can revert the process or any strategy that can stimulate the NrF2 pathway should bring about an amelioration in the damages caused by the ROS getting generated in the stressed tissues. However, it should be noted here that the oxidative defence mechanism is a nutrient consuming process (Figure 4). This is a vicious cycle since when exposed to heat stress several nutrient co transporter genes get downregulated on one hand (discussed above) and the oxidative damages caused to the mucosal membrane of the small intestine do not allow sufficient nutrient uptake to take place (Figure 5). The overall result becomes a depressed feed intake and poor feed conversion efficiency (Figure 6).
Management of stress by internal defence systems
In chickens, a defence system is engaged to protect the body from the harmful effects of heat stress. HSP70 and HSP90 are members of the heat shock protein (HSP) family, which is the most conserved and well-studied genes, and their expression is altered by several substances or stressors (Xieet al., 2014). These two proteins are well-known elements of the body’s stress-resistance system. HSP70 protects the organism from the harmful consequences of oxidative stress (Favatieret al., 1997), whereas HSP90 interacts with client proteins during the last stages of folding and alters their shape (Wegele et al., 2004). Heat preconditioning is linked to the expression of these genes and an up regulation of these molecules enhances cell stability and develops thermotolerance during heat-stress conditions. Over-expression of certain HSPs is responsible for precipitating irreversible damages in the mucosal lining of small intestine leading to poor absorption of nutrients and decreased body weight. Exposure to high temperatures causes several defects in the intestinal epithelium, including damage to villus length and tip integrity, and increased cell shedding, which is thought to reduce epithelial barrier functions and increase vulnerability to bacterial translocation. There is a growing trend of the therapeutic targeting of HSPs via nutritional and pharmacological agents towards alleviating heat stress in commercial chicken. This is intriguing since generally it is postulated that an upregulated expression of the HSP genes should make the birds toned to combat heat stress. However, it is also true that an early up regulated expression to a milder extent followed by a down regulation of the same may be an effective strategy to reduce long standing damages caused by heat stress. Some plant-derived and pharmacological compounds with biological activities lead to the peak induction of HSPs at the time of stress with sustained induction for the first few days post-insult, leading to rapid cyto-protection, while other compounds attenuate the heat-stress-induced over expression of HSPs through their biological (antioxidant and anti-inflammatory)properties.
Molecular biology approaches for mitigation and management of heat stress
The poultry industry is continuously working on a variety of approaches for sustaining the thermal tolerance of chickens and reducing the harmful effects of heat stress. Recent technical advancements and the strategic application of molecular biology methodologies have allowed for a faster understanding of these difficulties and a more efficient response to them. It is feasible to gain profound insights into the control of food intake in heat-stressed chickens by analysing the gene expression of macronutrient transporters. The roles of vitamins and minerals in alleviating heat stress may be assessed for a better understanding of this issue. A better understanding of how different molecules and their interactions play role infighting stress in birds could be useful for the development of new approaches to improving poultry welfare and production by making birds less susceptible to damage caused by heat stress.
Conclusions
To improve livestock production, heat stress is one of the biggest challenges the poultry industry is faced with. Although reduced feed intake and body weight have been the key indicators of heat stress, comprehensive insights into the problem can be gained by examining the gene expression of macronutrient transporters, gastrointestinal health, and immune genes. Overall, heat stress has a significant impact that may be measured using the essential genes chosen as markers. Recent technology advancements and the strategic application of molecular biology technologies may enable us to quickly comprehend these difficulties and respond to them more effectively.
Arpana Verma Mukherjee and Sudipto Haldar, Agrivet Research and Advisory

